HOT TOPICS LIST
- Strategies
- Stocks
- Buy
- Investing
- Brokers
- Psychology
- Interviews
- Accumulate
- Sell
- Hold
- Spotlight
- Websites
- Candlestick Corner
- Gold & Metals
- Options Trading
LIST OF TOPICS
MUTUAL FUNDS
Biotech To The Future!
05/01/01 11:21:50 AM PSTby David Penn
With gene-based medicines, tissue regeneration, and cutting-edge treatments for cancer and HIV, biotechnology promises a revolution in medicine. Are there opportunities for investors as well?
| Biotechnology. When you hear that word, what comes to mind? Test-tube babies? Sheep clones named Dolly? Heated political debates over stem cell research? If one thing is clear, it's that biotechnology is far from some new-technology fad looking to capitalize on a current fascination with gadgets, toys, and colorful plastic devices. In fact, as Evan McCulloch, portfolio manager of the Franklin Biotechnology Discovery Fund notes, while the completion of the Human Genome Project in 2000 is widely and correctly seen as the landmark event in biotechnology, scientists would not even have been able to conceive of such a project had it not been for James Watson and Francis Crick's discovery of the double-helix nature of Dna in 1953. What makes biotechnology stocks so tempting to investors? Sharon Seiler, a biotechnology analyst with specialty investment bank Punk, Ziegel & Co., says that biotechnology is founded on the principle that "you could take rational biological approaches to drug development." This approach contrasts with the traditional trial-and-error process that pharmaceutical companies have pursued for decades, although the rise of biotechnology has helped many pharmaceutical companies adopt more "biologic" methods in their own drug development process. And while many of these biologic approaches began with things such as protein therapeutics (treatment based on the use of blood plasma, for example), biotechnology now includes the search for actual biological targets (places in the body where chemicals or biologic agents effect change at the molecular level). It is this search that gives so many researchers, doctors, and patients the hope that biotechnology may move medicine from mostly treating diseases to actually anticipating and preventing diseases before they strike.
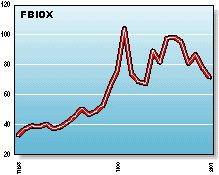
FBIOX: The Fidelity Select Biotechnology Fund invests in larger,
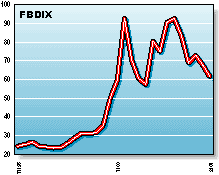
FBDIX: The Franklin Biotechnology Discovery Fund invests in many
This is also what has driven so many investors - individual and institutional, venture capital and retail - toward the biotechnology sector. As Bill Snider, general partner and cofounder of Emerging Technology Partners, a venture capital firm specializing in genomic companies notes, "The point of all this research and discovery is to come up with a compound you can patent as a drug and get a temporary monopoly to sell it." It is the possibility of hundreds of little monopolies on drugs that treat everything from the flu and Hiv to cancer and debilitating genetic disorders that has dollar signs flashing in the eyes of those who understand the huge profit potential of biotechnology. But it is potential only, not a certainty; as many analysts, money managers, and others familiar with the growing biotechnology industry insist, savvy investing in biotechnology companies is no mean feat for stock market newbies. Adds Snider, "[People] don't have a way to truly verify whether certain advancements in biotechnology are real. ... Ford can hype up a car," he explains, but it's only after potential customers test-drive it that the results come in. "In this field, it's not possible to do that in a mainstream way," he maintains. Evan McCulloch is even more emphatic: He believes biotechnology is an "extremely difficult sector for the average investor" to invest in. BIOTECH-BOUND What makes biotechnology such a tricky arena for the average investor? Most obviously, the (necessarily) abundant scientific discourse is one factor that can make it trying for the average layperson to understand just what any individual biotechnology company is up to. As difficult as it might have been for investors to understand microprocessors, network routers, and fiber optics, those concepts are virtually grammar-school primers compared to terms such as immunoglobulin, viral entry inhibitors, and phase II clinical trials. The biotechnological learning curve is made all the more problematic by the hype that often surrounds this form of research. In the same way that a new wireless solution or a new processing benchmark is cause for celebration and bullishness on the part of technology investors, a headline heralding "Cancer cure?" sends many biotech investors running for their brokers, often before attempting to read the rest of the story. Talking about the susceptibility of the biotech industry to media hype, Seiler laughs: "If I had a penny for every drug that cured tumors in mice, I'd be basking on a beach in Aruba." Seiler sees the possibility of superior treatments for maladies such as cancer and HIV as especially beguiling to many - both patients and investors - desperately longing for a blockbuster wonder drug. Noting oncology studies, for example, Seiler admits there is a great deal of anecdotal evidence in support of various treatments. But often those results fall apart when a real controlled trial is done. "You have to be very careful," Seiler says, "when somebody tells you that [a drug] is effective. Effective based on what data?" Tom Porter, general partner and cofounder of EDF Ventures, a venture capital firm with significant investments in a number of biotechnology companies, including GenVec and Selective Genetics, opines that one of the best ways for investors to learn about an arena as complicated as biotechnology might be to spend less time on the science page of the local newspaper and more time reading analysts' reports. "Find out who the best biotech analysts are," he suggests. As far as Porter is concerned, the best biotechnology analysts are capable of discussing both specific companies as well as the biotechnology sector as a whole. "And they do this in plain, everyday language that investors need to be able to read and understand." BIOTECH BASICS Generally, biotechnology products go through a regular series of steps from inception to market. A preclinical phase of research, testing, and discovery is followed by a series of clinical trials if the earliest experiments go well. There are three phases to the clinical trials. Phase I is generally a "safety-only" test during which the company seeks to ascertain the effects of the drug on a limited number of subjects. Both general safety and the possibility and extent of any side effects must be uncovered here. If the Federal Food and Drug Administration (FDA) decides that the drug is generally safe after a review of the phase I data, a phase II trial is held. Here, more specific effectiveness is judged on a larger group of patients than in phase I. Phase III is considered by some to be the "make or break" point in the drug approval process. General safety having been established in phase I and a satisfactory level of effectiveness in phase II on a larger, but still limited subject group in a clinical setting, phase III trials involve both a larger test subject group as well as expanded criteria, such as the improvement in the patient's overall life and health condition outside of the clinic. It is usually after a successful phase III test that a drug will be guaranteed at least a fair hearing before the FDA advisory board. 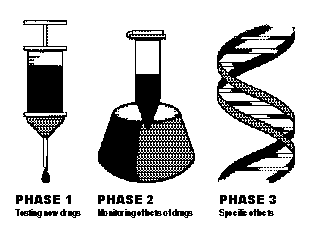
Investors must carefully evaluate the results of successful clinical trials before committing capital to biotechnology companies. A good phase II trial is only cause for celebration if it is followed by a successful phase III trial. And a successful phase III trial can be almost completely ruined by a poorly timed or poorly executed market launch strategy. Snider notes that "the FDA is very careful before they allow people to put drugs on the market ... [the FDA's] process is to be as efficient as possible." He notes that "the main objective of the FDA is safety," but he is somewhat concerned about the length of time the process takes: "[The time] is a big deal. [The FDA] does need to be faster because it does take a long time and cost a lot of money and can prevent a lot of things from being developed." Seiler is a bit more sanguine on the length of time that the FDA takes, stating that the FDA is "quicker than it used to be" and suggesting that the dramatic rise in applications for new clinical trials may have caught the agency understaffed. Seiler also provides another possible reason for complaints about the FDA: "It's easier for [companies] to say, OWell, we had this great drug and we did great trials and the FDA doesn't want to approve it,' than to say OWell, our drug had marginal effect and we really did poorly in our clinical trials, and now the FDA doesn't want to approve it.'" CHOOSING AMONG BIOTECHS What should individual investors look for in biotechnology companies? Analysts, venture capitalists, and biotech mutual fund managers all seem to agree that biotech companies need cash - and lots of it. Eli Neusner, director of research for MetaMarkets.com, notes that many biotechs, especially the smaller ones, spend "years plugging away" in research and development before ever bringing a single product to market. As such, biotechnology companies need a "large cash position" in order to "weather out" the time between research and development and profitability. Brian Younger, portfolio manager of the Fidelity Select Biotechnology Fund, agrees that "lots of cash" is vital to biotech companies. But Younger also wants to see accelerating revenue and earnings growth, particularly from the established, already-profitable biotechnology companies. He draws a sharp distinction between the established biotechs, such as Amgen (AMGN), Biogen (BGEN), and Immunex (IMNX), and some of the newer, "earlier-stage" biotechnology companies that may have few products in the pipeline and even fewer, if any, in the marketplace. Younger strongly cautions individual investors to avoid these smaller earlier-stage biotechs about which little may be known and for which no track record of successful clinical trials and/or product launches may exist. Savvy management is another major factor to look for in biotech companies. Says Snider: "You have to span the bridge of both science and business. Ideally, you're looking for a management team that has both a reasonably long history of business experience and also [has] some first-class scientists involved. You cannot be an effective company in this field without top science. And at the same time, just having top science doesn't make a good investment." McCulloch adds he likes to see managers with background in pharmaceutical work, as well as experience in managing large companies. As such, he is comfortable with the traditional corporate management structure - with chief financial officer (CFO), chief operating officer (COO), and chief executive officer (CEO) - with a chief science officer who is often the founder of the company and has stepped aside from day-to-day leadership to focus on the company's research and development.
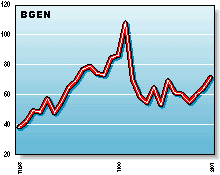
BGEN: Biogen has attracted increased attention with their psoriasis
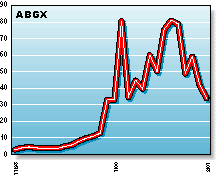
ABGX: Abgenix's xenomouse technology uses genetically engineered
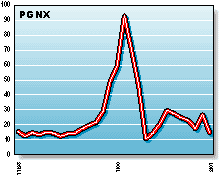
PGNX: Progenics Pharmaceuticals is developing
As if having good competitive products and effective managerial leadership were not difficult enough, Seiler suggests that there are certain conditions for which it is more difficult to develop drugs. As someone who tends to follow companies developing treatments for cancer and infectious disease, Seiler is more comfortable with companies whose clinical trials are likely to have very well-defined endpoints and benchmarks. "If you are doing a vaccine trial or a trial of an antiviral or an antibiotic or an antifungal, everybody agrees what the endpoint should be. ... You want to look for a clear, clinical route to market, a well-defined condition where people agree on what the trial results should be." Seiler contrasts this with areas such as wound-healing, commenting, "There have been a thousand products in development for wound-healing and it's not necessarily that products don't work, it's just very hard to show that they do work. Do you measure how quickly the wound heals? How fully it heals? Some combination of both?" The issue of the product pipeline is also a daunting one. If a company only has one or two products in development or on the market, then the risks of that company being edged out by competitors and having no other products as backup are far greater. Suggests Seiler, "One way to invest in biotech that's not quite so risky is to look for companies that have a portfolio of products and aren't dependent on the success of just one." BIOTECH FUNDS INSTEAD? A less risky way to invest in biotechnology is to avoid individual biotech companies altogether and invest in biotech mutual funds instead. Both Evan McCulloch of Franklin and Brian Younger of Fidelity make this point with some conviction. Citing the fact that biotechnology is "easily hyped," Younger points out that, unlike many other mutual funds (including other sector funds), there is a real advantage to knowledge when investing with a biotechnology mutual fund, a premium not present when the average investor tries to make his or her own investments. "You can really lose all of your money by [just] owning one biotech stock," argues Younger. "Whereas in a biotech mutual fund, that's just not going to happen." In terms of questions to ask when selecting among biotechnology mutual funds, both McCulloch and Younger strike similar chords:
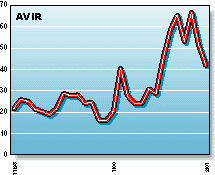
AVIR: Aviron's best-known product is their
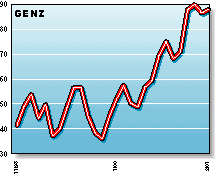
GENZ: Considered a leading biotechnology company, Genzyme
Both managers believe investing with a large, established mutual fund family has enormous benefits when investing in a sector like biotechnology. A fund family with a reputation to uphold is unlikely to risk its reputation on a fly-by-night sector fund that the company doesn't really believe in and is unwilling to support with top-quality analyst and managerial talent. Adds Younger, "There are a lot of new biotech funds out there from companies I've never even heard of." BELIEVING IN BIOTECH On the long-term prognosis for biotechnology, analysts, venture capitalists, and money managers seem universally upbeat. McCulloch says that there were 33 profitable biotech firms in 2000 and there will be an estimated 67 profitable ones by 2002. This is why McCulloch suggests that "every year [we] have to look at the sector as a new one." While acknowledging the rough time that biotechnology stocks had in the first part of 2000 and in the first few months of 2001, Younger notes that biotechnology - unlike the Nasdaq, for example - was up in 2000. "The near term," he says, "is a different issue" from the long term, citing the overall negative sentiment of the stock market in general and the Nasdaq in particular. Although biotechnology has fundamentals separate from those of other technology areas such as semiconductors or fiberoptics, it can be difficult for biotechs to escape the downward pull of an overall market decline.
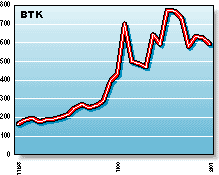
BTK: The Amex Biotechnology Index helps investors
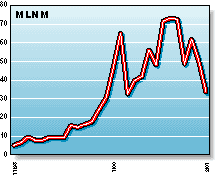
MLNM: Millennium Pharmaceuticals specializes in treatments
Another reason to consider the biotechnology sector optimistically has to do with the sector's customers. Tom Porter remarks that while it depends on the specific product or service being provided, academic institutions, government research institutes, and other nonprofit research laboratories are among biotech's biggest customers. Beyond these nonprofit entities, pharmaceutical companies make up a significant number of those buying biotechnology solutions. "The research market," marvels Porter, "is just huge." Porter also thinks the development of support and ancillary services for biotechnology companies will only add to the overall positive outlook for the sector. Whether these services come in the form of new and more robust software to handle the enormous amounts of data involved in biotech research, or medical devices to improve the delivery of biotech medicines, or even new specialized facilities to conduct more unique biotech treatments and therapies, Porter sees the growth of these other entities as significant to the average investor. In fact, he likens investment in some of these ancillary companies to selling picks and shovels during the California Gold Rush instead of joining the hordes panning for gold. Finally, biotechnology companies tend not to have the same pricing pressure that pharmaceutical companies do. This makes the sector much more resilient in the face of declines in consumer spending or confidence compared to other sectors. While successful pharmaceutical drugs provide antibalding medications, new antihistamines, and remedies for conditions such as blood pressure, biotechnology drugs are more often associated with maladies such as cancer, viral infections, and genetic disorders. As Seiler put it, "Drugs for cancer or HIV are not discretionary purchases." David Penn can be reached at DPenn@Traders.com. Copyright © 2001 Technical Analysis, Inc. All rights reserved.
|
Technical Writer for Technical Analysis of STOCKS & COMMODITIES magazine, Working-Money.com, and Traders.com Advantage.
| Title: | Traders.com Technical Writer |
| Company: | Technical Analysis, Inc. |
| Address: | 4757 California Avenue SW |
| Seattle, WA 98116 | |
| Phone # for sales: | 206 938 0570 |
| Fax: | 206 938 1307 |
| Website: | www.traders.com |
| E-mail address: | DPenn@traders.com |
Traders' Resource Links | |
| Charting the Stock Market: The Wyckoff Method -- Books | |
| Working-Money.com -- Online Trading Services | |
| Traders.com Advantage -- Online Trading Services | |
| Technical Analysis of Stocks & Commodities -- Publications and Newsletters | |
| Working Money, at Working-Money.com -- Publications and Newsletters | |
| Traders.com Advantage -- Publications and Newsletters | |
| Professional Traders Starter Kit -- Software | |
PRINT THIS ARTICLE

Request Information From Our Sponsors
- StockCharts.com, Inc.
- Candle Patterns
- Candlestick Charting Explained
- Intermarket Technical Analysis
- John Murphy on Chart Analysis
- John Murphy's Chart Pattern Recognition
- John Murphy's Market Message
- MurphyExplainsMarketAnalysis-Intermarket Analysis
- MurphyExplainsMarketAnalysis-Visual Analysis
- StockCharts.com
- Technical Analysis of the Financial Markets
- The Visual Investor
- VectorVest, Inc.
- Executive Premier Workshop
- One-Day Options Course
- OptionsPro
- Retirement Income Workshop
- Sure-Fire Trading Systems (VectorVest, Inc.)
- Trading as a Business Workshop
- VectorVest 7 EOD
- VectorVest 7 RealTime/IntraDay
- VectorVest AutoTester
- VectorVest Educational Services
- VectorVest OnLine
- VectorVest Options Analyzer
- VectorVest ProGraphics v6.0
- VectorVest ProTrader 7
- VectorVest RealTime Derby Tool
- VectorVest Simulator
- VectorVest Variator
- VectorVest Watchdog